Abstract
Background: So far, planning of clinical trials in multiple myeloma (MM) has been based on traditional outcome measures such as progression free (PFS) and overall survival (OS). However, treatment with drugs, which prolong survival, usually impacts quality of life (QoL). A good example is lenalidomide (LEN) maintenance therapy in transplant eligible newly diagnosed MM. While LEN is the only approved drug for maintenance therapy, clearly and consistently prolonging PFS in a number of trials, treatment comes with an increased risk for infections, secondary malignancies, fatigue and diarrhea. One consequence could be to define QoL as an additional study endpoint but the MM patient's perspective on this topic has not been systematically investigated yet.
Methods: To address this, we performed an online survey with MM patients with previous lenalidomide (LEN) maintenance. The survey contained a total of 205 questions, including the validated QoL C30- and My20-questionnary and questions pertaining the patient's preference for either prolonged survival or increased QoL. To assess differences between patient subgroups we used statistical analyses such as a random decision forest.
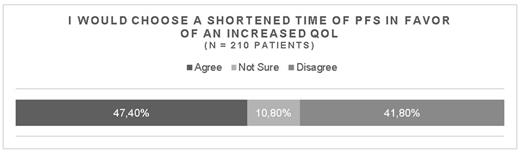
Results: 210 patients with MM completed the online survey within 48 days. 92 (47%) of patients chose a shortened time of PFS in favor of an increased QoL (QoL-group). An almost equal number of patients 81 (42 %) chose the exact opposite option (PFS-group). 11% of patients were unable to make this kind of decision. Thus, a sole measurement of the endpoint PFS would disregard the preference of ~1/2 of the patients. We used an unbiased artificial intelligence supported statistical approach to explore additional features of the QoL- and the PFS-group. Interestingly, the top variable associated with the QoL-group was pain related to bone disease at the time of diagnosis (P = 0.01). There was a trend for more advanced treatment lines in patients in the QoL-group compared to the PFS-group. Patients who preferred PFS were found to be more likely to hand over responsibility to their physicians.
Conclusion: We demonstrate that MM patients can be divided into two nearly equally sized groups, where one group favors PFS and the other one prefers QoL, with bone pain at diagnosis and the number of previous treatment lines impacting the decision. Since our results suggest that survival as the only endpoint for maintenance therapy studies in MM should be reconsidered, we propose PFS and QoL measures as co-primary endpoints to account for the heterogeneity in patients´ preferences and to collect the information necessary for shared decision-making in future patients.
Einsele: Janssen, Celgene/BMS, Amgen, GSK, Sanofi: Consultancy, Honoraria, Research Funding. Weinhold: Sanofi: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal